The Shape of the Ammonia Molecule Nh3 Is
Hybridization in Ammonia NH3 Molecule. 10 Does NH3 have dipole dipole forces.

Nh3 Molecular Geometry Shape And Bond Angles Ammonia Youtube
Si-28 mass 280 amu.
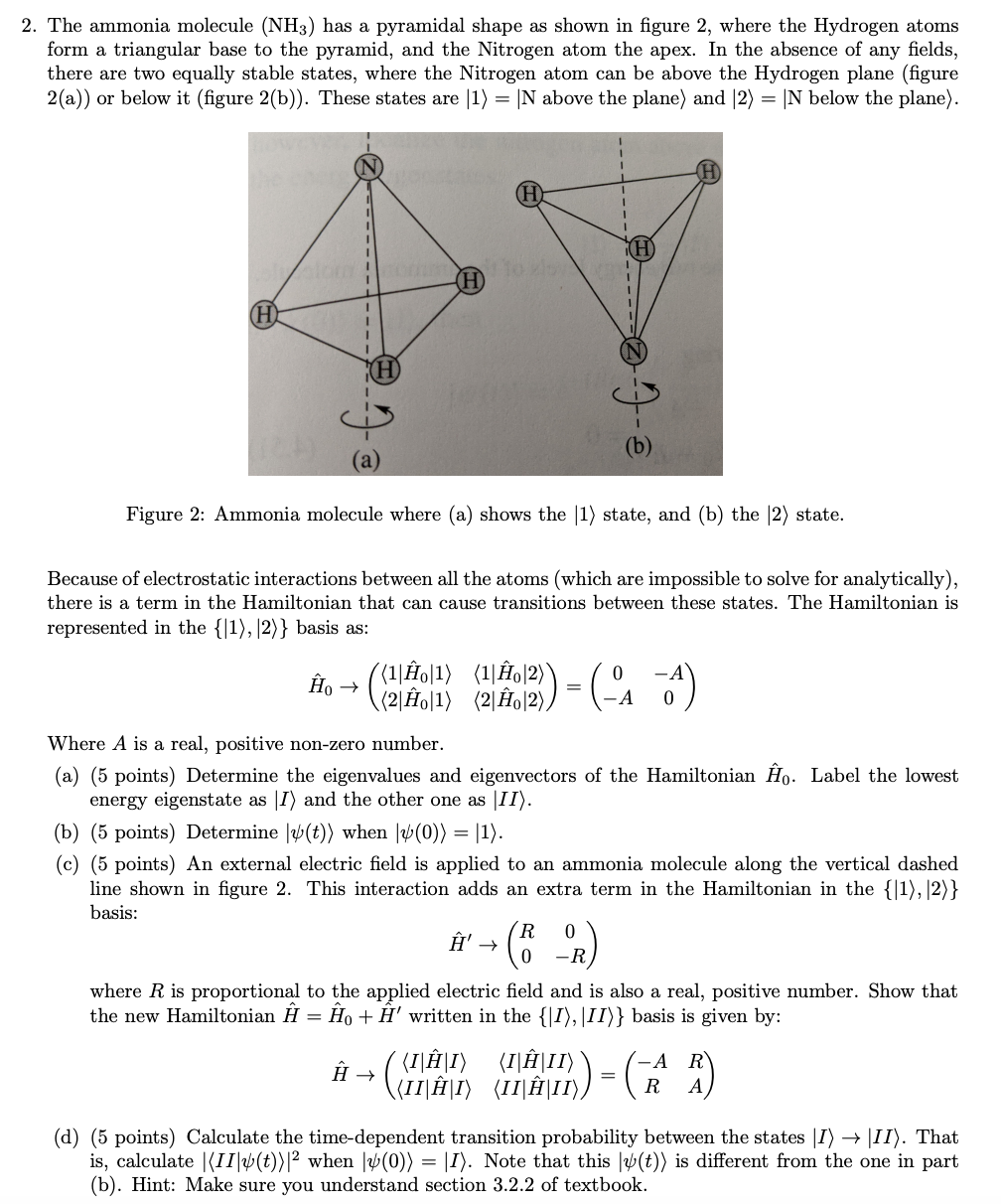
. In its concentrated form it is dangerous and caustic. Ammonia is NH3 and if you consider its hybridisation its sp3 thus its pyramidal. So your answer is B linear.
Does NH3 have 3 electron domains. 4 Is NH3 a hydrogen bond. 6 What type of solid does NH3 form.
5 Is NH3 strongest intermolecular force. 9 rows The molecular geometry or shape of NH 3 is a Trigonal pyramidal. In its aqueous form it is called ammonium hydroxide.
The shape of the ammonia molecule NH3 is. Geometry of NH3 is tetrahedral but as lone pairs are not considered in shape so its pyramidal. Due to the original pyramidal shape of the Ammonia molecule it is polar in nature as its atoms share unequal charges.
The NH3 molecular geometry molecular shape is trigonal pyramidal. When there is a single electron pair and three bond pairs the resulting molecular geometry is trigonal-pyramidal eg NH3. These are arranged in a tetrahedral shape.
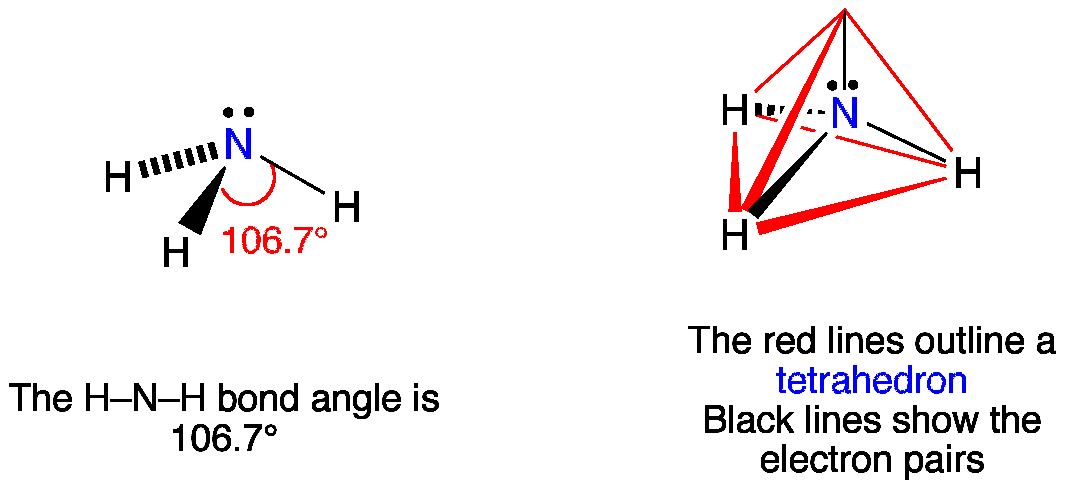
Ammonia or NH3 is a polar molecule as there is a large difference of electronegativities between Nitrogen and Hydrogen along with the asymmetric shape of the molecule. Let me explain why ammonia could either be trigonal pyramidal or tetrahedral and never trigonal planar. The molecule of ammonia has a trigonal pyramidal shape with angles of 1067 0.
Alinear bsquare cpyramidal doctagonal ehexagonal. 9 Is ammonia a hydrogen bond. The ammonia molecule has a trigonal pyramidal or distorted tetrahedral shape as predicted by the valence shell electron pair repulsion theory VSEPR theory with an experimentally determined bond angle of 1067.
Check out the valuable article already written on the polarity of ammonia. Ammonia has a tetrahedral molecular geometry. According to VSEPR theory the structure of the ammonia molecule NH3 is linear.
A sample of silicon has three naturally occurring isotopes. 3 What is the major intermolecular force in NH3. Both NH3 and NH4 ion have SP3 hybridization.
Shape should not be confused with geometry. The NH3 bond angles are 107 degrees because the hydrogen atoms are repelled by the lone pair of electrons on the Nitrogen atom. Tetrahedral By signing up youll get.
Ammonia is never a flat trigonal planar molecule. The shape of NH3 molecule is Trigonal pyramidal Its so because of the presence of a lone pair on Nitrogen N in ammonia. 8 Is NH3 ionic or covalent.
The shape of ammonia which has one nitrogen and three hydrogen atoms could be trigonal to begin with but be careful Ammonia is either trigonal pyramidal or tetrahedral. If the average atomic mass of silicon is 281 amu which isotope is the. The electron geometry of NH.
Atoms are held together by sharing electrons. Ammonia has 4 regions of electron density around the central nitrogen atom 3 bonds and one lone pair. The bond angle in a molecule of ammonia NH3 is 107 degrees so why when part of a transition metal complex is the bond angle 1095 degrees.
What is the molecular geometry of NH3 molecule. Ammonia is a colorless gas with a chemical formula NH 3. All the Hydrogen atoms are arranged symmetrically around the Nitrogen atom which forms the base and the two nonbonding electrons form the tip which makes the molecular geometry of NH3 trigonal pyramidal.
NH3 Molecular Geometry. This the dominant intermolecular force and results in a greater attraction between NH3 molecules than there is between PH3 molecules. This inorganic compound has a pungent smell.
Si-29 mass 290 amu and Si-30 mass 300 amu. What is the shape of the ammonia NH3 molecule. Chemistry questions and answers.
It consists of hydrogen and nitrogen. Although PH3 is a larger molecule with greater dispersion forces than ammonia NH3 has very polar N-H bonds leading to strong hydrogen bonding. In a molecule with covalent bonding.
11 What type of bond is NH3 polar or nonpolar. Keeping this in view what is the difference between the shape of nh3 and nh4 1. Part A The shape of the ammonia molecule NH3 is O tetrahedral O trigonal planar linear O bent O trigonal pyramidal My Answers Give Up Submit.
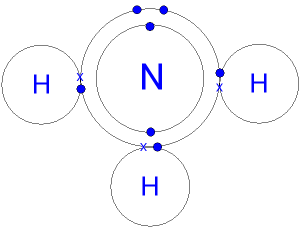
7 Does NH3 have intermolecular hydrogen bonding. The bond between each nitrogen and hydrogen atom is covalent and made up of sigma σ bonds only and no pi π. The central nitrogen atom has five outer electrons with an additional electron from each hydrogen atom.
The uneven dispersion of electric charges in the molecule makes it a polar molecule. The resulting molecular shape is trigonal pyramidal with H-N-H angles of 1067. The shape of the ammonia molecule NH3 is.
If all of these are bond pairs the molecular geometry is tetrahedral eg CH4. Use the arrangement of bonded atoms to determine the molecular geometry. A quick explanation of the molecular geometry of NH3 including a description of the NH3 bond angles.
What Is The Shape Of An Nh3 Molecule Quora

What Is The Shape Of The Ammonia Molecule Study Com

What Is The Shape Of Nh3 Molecule Diagram Brainly In
What Is The Shape Of An Nh3 Molecule Quora

2 The Ammonia Molecule Nh3 Has A Pyramidal Shape Chegg Com
How To Predict The Molecular Geometry Of Ammonia Based On The Vsepr Model Quora

Nh3 Ammonia Molecule Royalty Free Vector Image
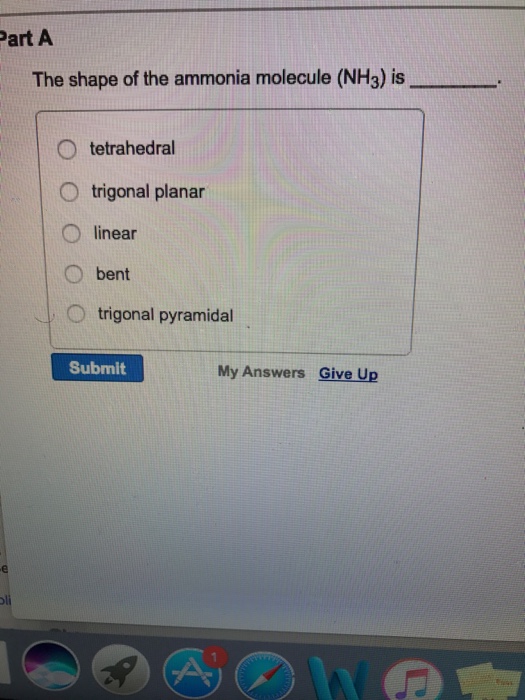
Solved Part A The Shape Of The Ammonia Molecule Nh3 Is O Chegg Com
What Is The Shape Of An Nh3 Molecule Quora
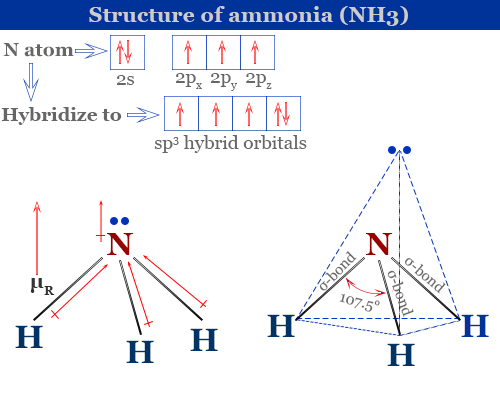
Ammonia Properties Structure Uses Production

Nh3 Molecular Geometry Science Education And Tutorials
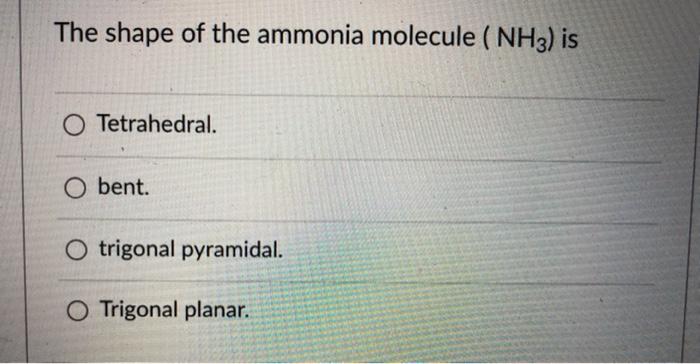
Solved The Shape Of The Ammonia Molecule Nh3 Is O Chegg Com
![]()
Ammonia Molecule Icon Stock Illustration Illustration Of Toxic 168347444

What Is The Shape Of The Ammonia Molecule Nh3 R Chemhelp

Molecular Structure Of Ammonia Nh3 Youtube

Nh3 Molecular Geometry Shape And Bond Angles Ammonia Youtube
Comments
Post a Comment